当前位置:首页 > 商业/管理/HR > 项目/工程管理 > 2羟丙基三甲基氯化铵壳聚糖的制备及其表征蔡照胜
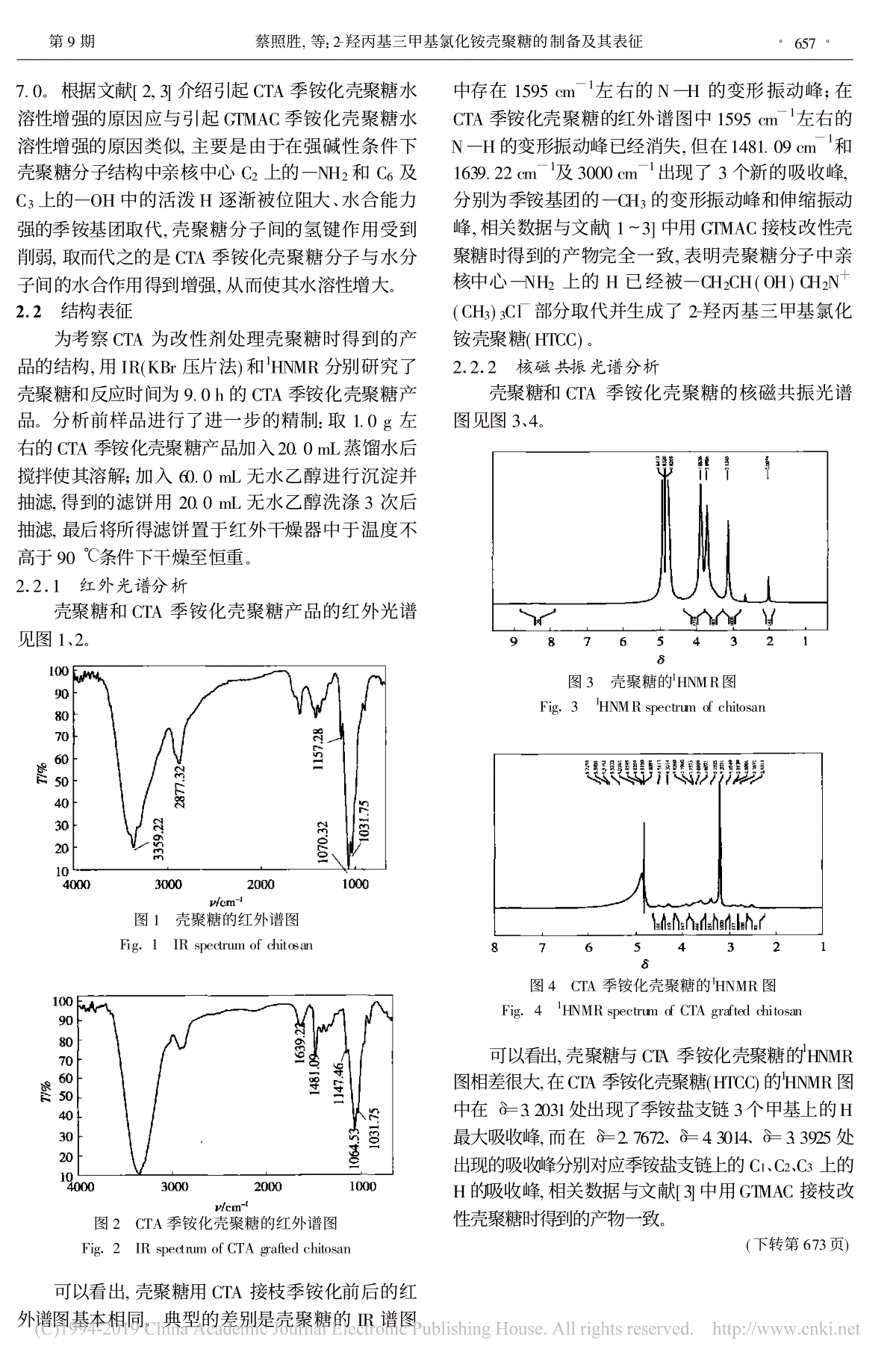
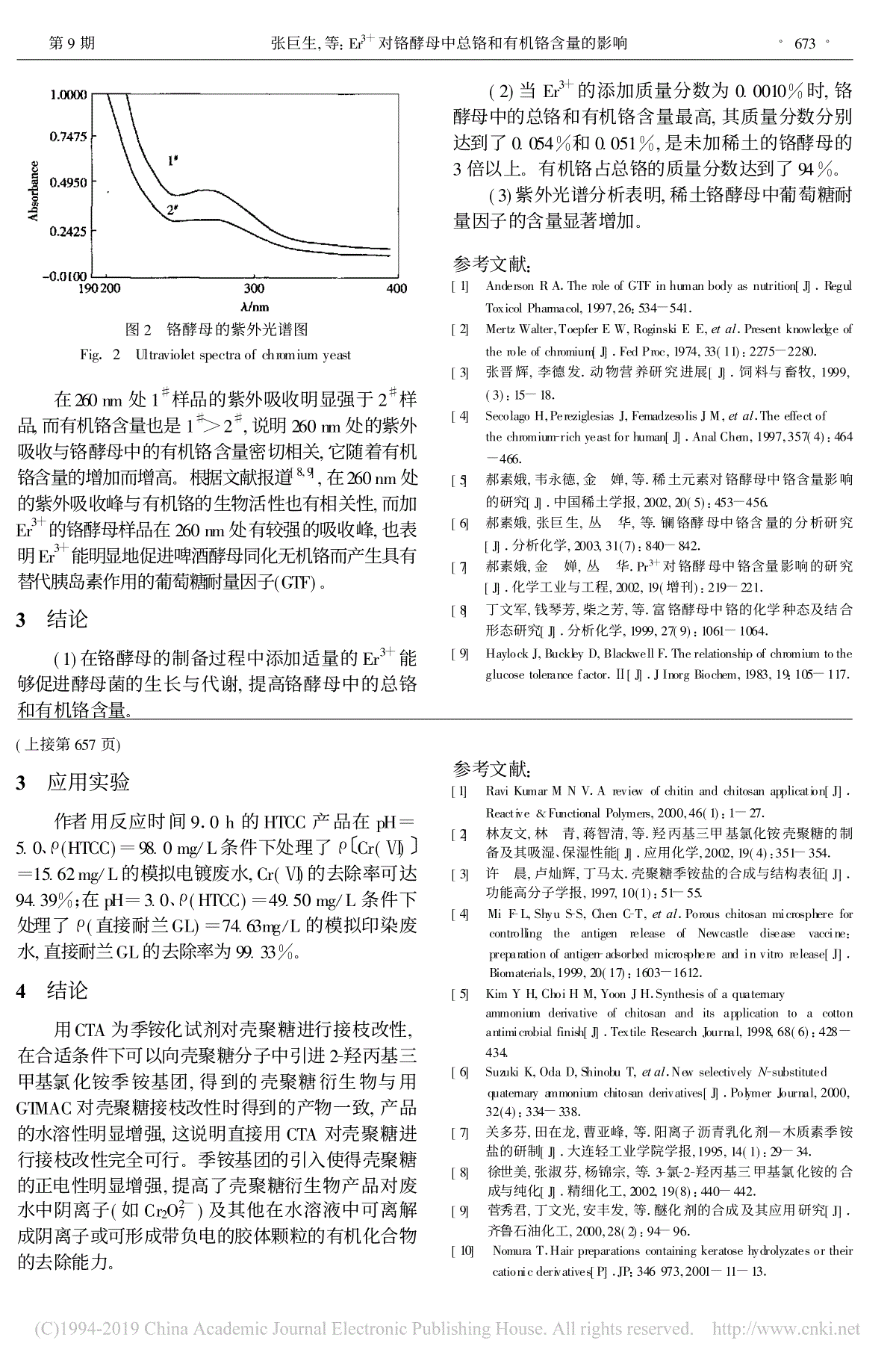
21920049精细化工FINECHEMICALSVol.21,No.9Sept.20042-蔡照胜1,2,王锦堂1*,杨春生2,许 琦2,严金龙2(1., 210009;2., 224003):90%(CTS),,w(NaOH)=40.0%,3--2-(CTA),m(NaOH)∶m(CTS)=1.0∶1.0,m(CTA)∶m(CTS)=4.0∶1.0,65.0℃2-(HTCC)。,9.0h,HTCC90.0%,pH=6.7~7.0w(HTCC)=3.0%。IR1HNMR,CTS。:(CTS);3--2-(CTA);;;2-(HTCC);:O636.1 :A :1003-5214(2004)09-0655-04PreparationandCharacterizationofChitosanGraftedwithCTACAIZhao-sheng1,2,WANGJin-tang1*,YANGChun-sheng2,XUQi2,YANJin-long2(1.SchoolofScience,NanjingUniversityofTechnology,Nanjing210009,Jiangsu,China;2.DepartmentofChemicalEngineering,YanchengInstituteofTechnology,Yancheng224003,Jiangsu,China)Abstract:2-Hydroxypropyltrimethylammoniumchloridechitosan(HTCC)waspreparedusingchitosan(CTS)withdegreeofdeacetylationof90.0%asrawmaterial,2-propanolassolvent,40.0%aqueousNaOHsolutionascatalystand3-chloro-2-hydroxypropyltrimethylammoniumchloride(CTA)asgraftingagent,whenm(NaOH)∶m(CTS)=1.0∶1.0,m(CTA)∶m(CTS)=4.0∶1.0,andreactiontemperaturewas65.0℃.Thegraftrateofproductcouldexcess90.0%whenreactiontimewasuptoormorethan9.0h.TheproductcoulddissolveindistilledwaterofpH6.7~7.0toformaw(HTCC)=3.0%aqueoussolution.IRand1HNMRshowedthatthegraftreactionhappenedmainlyontheaminogroupofCTS.Keywords:chitosan;3-chloro-2-hydroxypropyltrimethylammoniumchloride;graftmodification;quaternaryammoniumsaltgraftedchitosan;2-hydroxypropyltrimethylammoniumchloridechitosan;graftrateFoundationitem:FundofenvironmentalprotectionofJiangsuprovince(2002031) (CTS),,。pH3~4,,30[1]。CTS,(,GTMAC),GTMACCTSH[2~6]。GTMAC2,3-:2004-01-01:(2002031):(1968-),,,,,2002,,:0515-8335195,E-mail:jsyc_czs@sohu.com,jsyc_czs@163.com。:,,,,:025-83314900。DOI:10.13550/j.jxhg.2004.09.005CTS,、CTS,CTS,[7]。3--2-(CTA)GTMAC,GTMAC[7],、[7~10],,GTMAC、,CTA,CTS2-(HTCC)。1 1.1 、1.1.1 原料与试剂3--2-(CTA,):w(CTA)=49.7%;:(≥90%),,;NaOH:GR,;:AR,;:AR,;:AR,;:AR,;:AR,。1.1.2 仪器:FA1604,;:NEXUS670(NicoletU.S.A);:CH1832;:BRUKERDRX500;:WS70-1,。1.2 CTA250mL4.0g,4.0gNaOH6.0mLw(NaOH)=40.0%;50.0mL,55.0℃,4.0h60.0℃,CTA32.0mL,65.0℃,,65.0℃;w(HCl)=10.0%pH=7.0;w()=85.0%60.0mL(3);50.0mL(3);90℃,CTAHTCC。。1.3 CTA1.0gCTA。0.500gHTCC。50mLw(HTCC)=1.0%,0.1000mol/L1.00mLHTCC;0.1000mol/LAgNO3,HTCCCl-,:X(%)=(c1V1-c0V0)/[m-(c1V1-c0V0)×151.6]×161.2×100:c1—HTCCCl-,mol/L;c0—HTCC,mol/L;V1—HTCC,L;VO—HTCC,L;m—HTCC,g;161.2—;151.6—。1.4 0.300gHTCC10.0mL,;0.1000mol/LpHpH。2 2.1 CTACTA1。1 Table1 Therelationshipofreactiontime,graftrateandwater-solubilityt/hHTCC/gHTCCc(Cl-)/(mol/L)/%HTCCpH12.00.49120.0186337.24.224.00.50830.0255059.34.736.00.48970.0282075.25.447.00.51060.0309082.96.058.00.49660.0311588.66.369.00.48760.0315093.76.7710.00.50950.0335397.87.0811.00.49820.03341101.47.0912.00.51870.03534105.37.0,HTCC,:,,HTCC;90.0%HTCC;HTCCpH·656·精细化工 FINECHEMICALS 21 7.0。[2,3]CTAGTMAC,C2—NH2C6C3—OHH、,,CTA,。2.2 CTA,IR(KBr)1HNMR9.0hCTA。:1.0gCTA20.0mL;60.0mL,20.0mL3,90℃。2.2.1 红外光谱分析CTA1、2。1 Fig.1 IRspectrumofchitosan2 CTAFig.2 IRspectrumofCTAgraftedchitosan,CTA。IR1595cm-1N—H;CTA1595cm-1N—H,1481.09cm-11639.22cm-13000cm-13,—CH3,[1~3]GTMAC,—NH2H—CH2CH(OH)CH2N+(CH3)3Cl-2-(HTCC)。2.2.2 核磁共振光谱分析CTA3、4。3 1HNMRFig.3 1HNMRspectrumofchitosan4 CTA1HNMRFig.4 1HNMRspectrumofCTAgraftedchitosan,CTA1HNMR,CTA(HTCC)1HNMRδ=3.20313H,δ=2.7672、δ=4.3014、δ=3.3925C1、C2、C3H,[3]GTMAC。(673)·657·9,:2-2 Fig.2 Ultravioletspectraofchromiumyeast260nm1#2#,1#2#,260nm,。[8,9],260nm,Er3+260nm,Er3+(GTF)。3 (1)Er3+,。 (2)Er3+0.0010%,,0.054%0.051%,3。94%。(3),。:[1] AndersonRA.TheroleofGTFinhumanbodyasnutrition[J].RegulToxicolPharmacol,1997,26:534-541.[2] MertzWalter,ToepferEW,RoginskiEE,etal.Presentknowledgeoftheroleofchromium[J].FedProc,1974,33(11):2275-2280.[3] ,.[J].,1999,(3):15-18.[4] SecolagoH,PereziglesiasJ,FemadzesolisJM,etal.Theeffectofthechromium-richyeastforhuman[J].AnalChem,1997,357(4):464-466.[5] ,, ,.[J].,2002,20(5):453-456.[6] ,, ,.[J].,2003,31(7):840-842.[7] , , .Pr3+[J].,2002,19():219-221.[8] ,,,.[J].,1999,27(9):1061-1064.[9] HaylockJ,BuckleyD,BlackwellF.Therelationshipofchromiumtotheglucosetolerancefactor.Ⅱ[J].JInorgBiochem,1983,19:105-117.(657)3 9.0hHTCCpH=5.0、ρ(HTCC)=98.0mg/Lρ〔Cr(Ⅵ)〕=15.62mg/L,Cr(Ⅵ)94.39%;pH=3.0、ρ(HTCC)=49.50mg/Lρ(GL)=74.63mg/L,GL99.33%。4 CTA,2-,GTMAC,,CTA。,(Cr2O2-7)。:[1] RaviKumarMNV.Areviewofchitinandchitosanapplication[J].Reactive&FunctionalPolymers,2000,46(1):1-27.[2] , ,,.、[J].,2002,19(4):351-354.[3] ,,.[J].,1997,10(1):51-55.[4] MiF-L,ShyuS-S,ChenC-T,etal.PorouschitosanmicrosphereforcontrollingtheantigenreleaseofNewcastlediseasevaccine:preparationofantigen-adsorbedmicrosphereandinvitrorelease[J].Biomaterials,1999,20(17):1603-1612.[5] KimYH,ChoiHM,YoonJH.Synthesisofaquaternaryammoniumderivativeofchitosananditsapplicationtoacottonantimicrobialfinish[J].TextileResearchJournal,1998,68(6):428-434.[6] SuzukiK,OdaD,ShinobuT,etal.NewselectivelyN-substitutedquaternaryammoniumchitosanderivatives[J].PolymerJournal,2000,32(4):334-338.[7] ,,,.—[J].,1995,14(1):29-34.[8] ,,,.3--2-[J].,2002,19(8):440-442.[9] ,,,.[J].,2000,28(2):94-96.[10] NomuraT.Hairpreparationscontainingkeratosehydrolyzatesortheircationicderivatives[P].JP:346973,2001-11-13.·673·9,:Er3+


 三七文档所有资源均是用户自行上传分享,仅供网友学习交流,未经上传用户书面授权,请勿作他用。
三七文档所有资源均是用户自行上传分享,仅供网友学习交流,未经上传用户书面授权,请勿作他用。
本文标题:2羟丙基三甲基氯化铵壳聚糖的制备及其表征蔡照胜
链接地址:https://www.777doc.com/doc-6525187 .html