当前位置:首页 > 商业/管理/HR > 管理学资料 > 超声波对活性炭吸附脱附Cr的影响荆国华
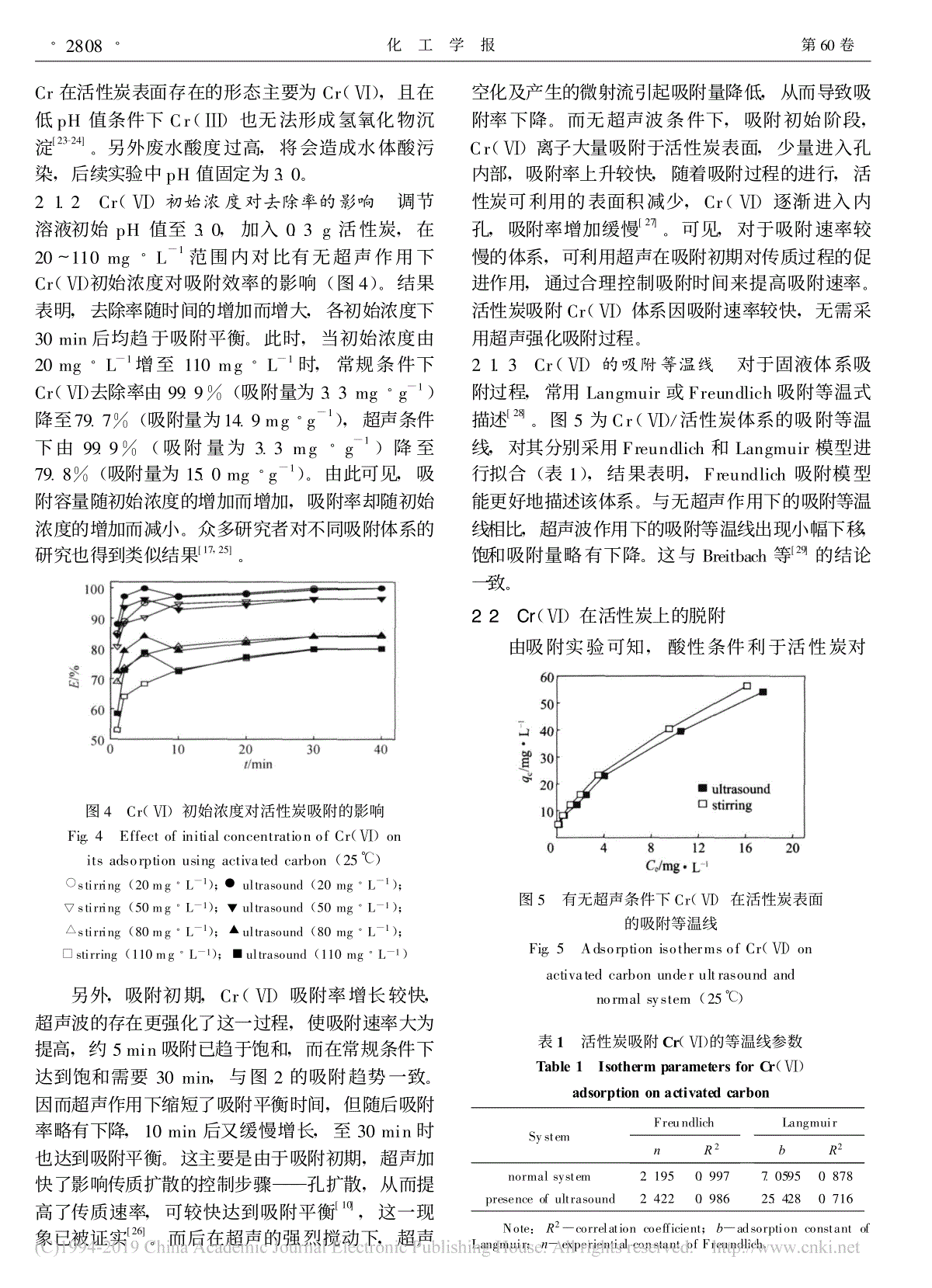
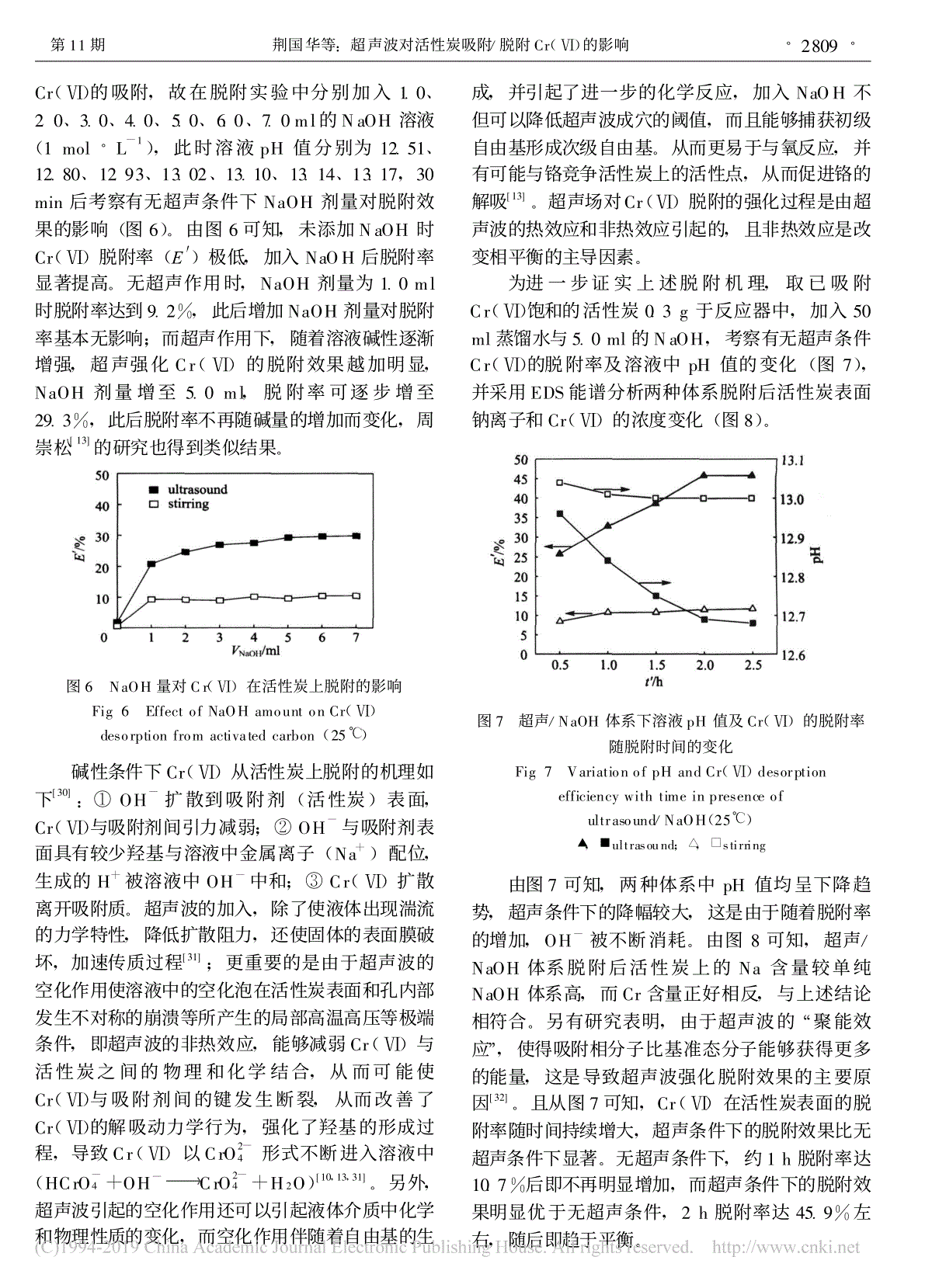
60 11 Vol.60 No.11 200911 CIESC Journal November 2009/Cr(Ⅵ)荆国华,董梅霞,周作明,许庆清(,361021):/Cr(Ⅵ),:,Cr(Ⅵ)pH,,,pH,;Cr(Ⅵ)20mg·L-1110mg·L-1,Cr(Ⅵ)99.9%79.8%,3.3mg·g-115.0mg·g-1,,,,。,,Cr(Ⅵ);NaOH,NaOH,NaOH。:;;;;:O647.3 :A:0438-1157(2009)11-2805-08Effectsofultrasoundonadsorptionanddesorptionofchromium(Ⅵ)onactivatedcarbonJINGGuohua,DONGMeixia,ZHOUZuoming,XUQingqing(CollegeofChemicalEngineering,HuaqiaoUniversity,Xiamen361021,Fujian,China)Abstract:Effectsofultrasoundontheadsorptionanddesorptionofchromium(Ⅵ)onactivatedcarbonwerestudied.Resultsshowedthatwithintherangeofexperimentconcerned,theadsorptionratioofchromium(Ⅵ)decreaseswithpHincreasingregardlessultrasoundapplication.However,withultrasound,theequilibriumofthesystemmovestothedirectioninthedecreaseofadsorption,whichbecomesseverewiththeincreaseinpH.Withultrasound,astheinitialCr(Ⅵ)concentrationincreasesfrom20mg·L-1to110mg·L-1,theremovalefficiencyofCr(Ⅵ)dropsfrom99.9%to79.8%,whiletheequilibriumadsorptioncapacityincreasesfrom3.3mg·g-1to15.0mg·g-1,whichissimilartothatwithoutultrasound.Theadsorptionratiocontinuouslyincreasesuntiltoanequilibriumwithoutultrasound,while,itappearsaslightdecreaseafterbeingquicklytotheequilibrium,andthengraduallyincreasestotheequilibriumagainincasewithultrasound.ThedesorptionratioofCr(Ⅵ)isverysmallindistilledwaterregardlessultrasoundapplication.While,thedesorptioncanbegreatlyimprovedbytheadditionofNaOH,andthispromotioneffectisremarkablefortheultrasonicsystem.Keywords:activatedcarbon;chromium(Ⅵ);ultrasound;adsorption;desorption 2009-02-08,2009-07-17。:(1975—),,,。:(D0710019);(07HZR22)。 Receiveddate:2009-02-08.Correspondingauthor:JINGGuohua,zhoujing@hqu.edu.cnFoundationitem:supportedbytheNaturalScienceFoundationofFujianProvince(D0710019)andtheNaturalScienceFoundationofHuaqiaoUniversity(07HZR22). 、、,,,Cr(Ⅵ),。,/,、、,、、,[1]。,,。/,Mohammad[2],,1.3~1.81.6~2.7;[3],,,。[4]/D301G。[5-9],,,。,,,;,,,[5]。,。Hamdaoui[10]/,。[11-12]。,。/。[13],,。Cr(Ⅵ),Cr(Ⅵ)//,,Cr(Ⅵ)/。1 1.1 ();(,1.4mol·L-1HCl24h,110℃)。(88-1,);-(UV-4802H,Unico);pH(Orion868,Themo);(S-3500N,);X(Inca,Oxford);JW-K()。1.2 :50mg·L-1Cr(Ⅵ),50ml,0.5mol·L-1HClNaOHpH,,(20kHz,0.7~0.8A),Cr(Ⅵ)。:,50mlCr(Ⅵ)100mg·L-1、pH3.0,,Cr(Ⅵ),,110℃50ml,NaOH,(),Cr(Ⅵ),。,。1。1 Fig.1 Deviceforultrasoundexperiment1.3 Cr(Ⅵ);·2806· 60 Cr-[14]。2 2.1 Cr(Ⅵ)2.1.1 pH值对吸附的影响 pH3.0、5.0、8.0、11.0,0.3g,pHCr(Ⅵ)(2)。,,pH,。pH11.03.0,30minCr(Ⅵ)(E)25.8%96.7%,12.2%96.9%。,pHCr(Ⅵ),pHCr(Ⅵ)。Sharma[15]Cr(Ⅵ)/。pH,H+[16-17],M+、MOH+2[18](M),Cr(Ⅵ)。,Cr(Ⅵ)HCrO-4、Cr2O2-7、CrO2-4,pH,。,Cr(Ⅵ)HCrO-4,,。pH,OH-,HCrO-4,,[16-17],Rao[19]Ucun[20]。,,,Cr(Ⅵ),,pHCr(Ⅵ),pH。pH=11.0,,Cr(Ⅵ)3.5%。-,-Cr(Ⅵ)[5,21]。Demiral[16],pH2,,Cr(Ⅵ)Cr(Ⅲ)。3C+2Cr2O2-7+16H+3CO2←+4Cr3++8H2O(1)2 pHCr(Ⅵ)Fig.2 EffectofinitialpHonCr(Ⅵ)adsorptionusingactivatedcarbon(25℃)◆stirring(pH=3.0);□ultrasound(pH=3.0);stirring(pH=5.0);ultrasound(pH=5.0);■stirring(pH=8.0);△ultrasound(pH=8.0);★stirring(pH=11.0);○ultrasound(pH=11.0) 3C+Cr2O2-7+8H+3CO←+2Cr3++4H2O(2)CrCr(Ⅵ),CrpH1.0,Cr(Ⅵ)Cr(3)。,,Cr(Ⅵ)Cr,Cr(Ⅵ)Cr(Ⅲ),Cr(Ⅲ),Cr。,Cr(Ⅵ),Cr,Cr(Ⅲ),,[22]。3 pH1.0CrCr(Ⅵ)Fig.3 RemovalrateoftotalchromiumandhexavalentchromiumunderpH=1.0(25℃)◆stirring(Cr6+);■ultrasound(Cr6+);□stirring(Crtotal);△ultrasound(Crtotal)2.2EDS·2807· 11 :/Cr(Ⅵ)CrCr(Ⅵ),pHCr(Ⅲ)[23-24]。,,pH3.0。2.1.2 Cr(Ⅵ)初始浓度对去除率的影响 pH3.0,0.3g,20~110mg·L-1Cr(Ⅵ)(4)。,,30min。,20mg·L-1110mg·L-1,Cr(Ⅵ)99.9%(3.3mg·g-1)79.7%(14.9mg·g-1),99.9%(3.3mg·g-1)79.8%(15.0mg·g-1)。,,。[17,25]。4 Cr(Ⅵ)Fig.4 EffectofinitialconcentrationofCr(Ⅵ)onitsadsorptionusingactivatedcarbon(25℃)★stirring(20mg·L-1);○ultrasound(20mg·L-1);stirring(50mg·L-1);ultrasound(50mg·L-1);■stirring(80mg·L-1);△ultrasound(80mg·L-1);◆stirring(110mg·L-1);□ultrasound(110mg·L-1) ,,Cr(Ⅵ),,,5min,30min,2。,,10min,30min。,———,,[10],[26]。,,。,,Cr(Ⅵ),,,,,Cr(Ⅵ),[27]。,,,。Cr(Ⅵ),。2.1.3 Cr(Ⅵ)的吸附等温线 ,LangmuirFreundlich[28]。5Cr(Ⅵ)/,FreundlichLangmuir(1),,Freundlich。,,。Breitbach[29]。2.2 Cr(Ⅵ),5 Cr(Ⅵ)Fig.5 AdsorptionisothermsofCr(Ⅵ)onactivatedcarbonunderultrasoundandnormalsystem(25℃) 1 Cr(Ⅵ)Table1 IsothermparametersforCr(Ⅵ)adsorptiononactivatedcarbonSystemFreundlichnR2LangmuirbR2normalsystem2.1950.9977.05950.878presenceofultrasound2.4220.98625.4280.716 Note:R2—correlationcoefficient;b—adsorptionconstantofLangmuir;n—experientialconstantofFreundlich.·2808· 60 Cr(Ⅵ),1.0、2.0、3.0、4.0、5.0、6.0、7.0mlNaOH(1mol·L-1),pH12.51、12.80、12.93、13.02、13.10、13.14、13.17,30minNaOH(6)。6,NaOHCr(Ⅵ)(E′),NaOH。,NaOH1.0ml9.2%,NaOH;,,Cr(Ⅵ),NaOH5.0ml,29.3%,,[13]。6 NaOHCr(Ⅵ)Fig.6 EffectofNaOHamountonCr(Ⅵ)desorptionfromactivatedcarbon(25℃) Cr(Ⅵ)[30]:①OH-(),Cr(Ⅵ);②OH-(Na+),H+OH-;③Cr(Ⅵ)。,,,,[31];,,Cr(Ⅵ),Cr(Ⅵ),Cr(Ⅵ),,Cr(Ⅵ)CrO2-4(HCrO-4+OH-CrO2-4+H2O)[10,13,31]。,,,,NaOH,。,,[13]。Cr(Ⅵ),。,Cr(Ⅵ)0.3g,50ml5.0mlNaOH,Cr(Ⅵ)pH(7),EDSCr(Ⅵ)(8)。7 /NaOHpHCr(Ⅵ)Fig.7 VariationofpHandCr(Ⅵ)desorptionefficiencywithtimeinpresenceofultrasound/NaOH(25℃)△,□ultrasound;■,◆stirring 7,pH,,,OH-。8,/NaOHNaNaOH,Cr,。,“”,,[32]。7,Cr(Ⅵ),。,1h10.7%,,2h45.9%,。·2809· 11 :/Cr(Ⅵ)8 EDSFig.8 EDSanalysisonactivatedcarbonsurfaceafterdesorption ,NaOH、2h1.4mol·L-1HCl(),50ml100mg·L-1Cr(Ⅵ),9。9 NaOHFig.9 EffectofNaOHamount



 三七文档所有资源均是用户自行上传分享,仅供网友学习交流,未经上传用户书面授权,请勿作他用。
三七文档所有资源均是用户自行上传分享,仅供网友学习交流,未经上传用户书面授权,请勿作他用。
本文标题:超声波对活性炭吸附脱附Cr的影响荆国华
链接地址:https://www.777doc.com/doc-6547369 .html